Evening friends
Every person we try to wake up goes through the same stages of denial and hopefully, eventually, acceptance. Some people we talk to really want to know everything you explain (absolutely understand, they have been lied to before!)
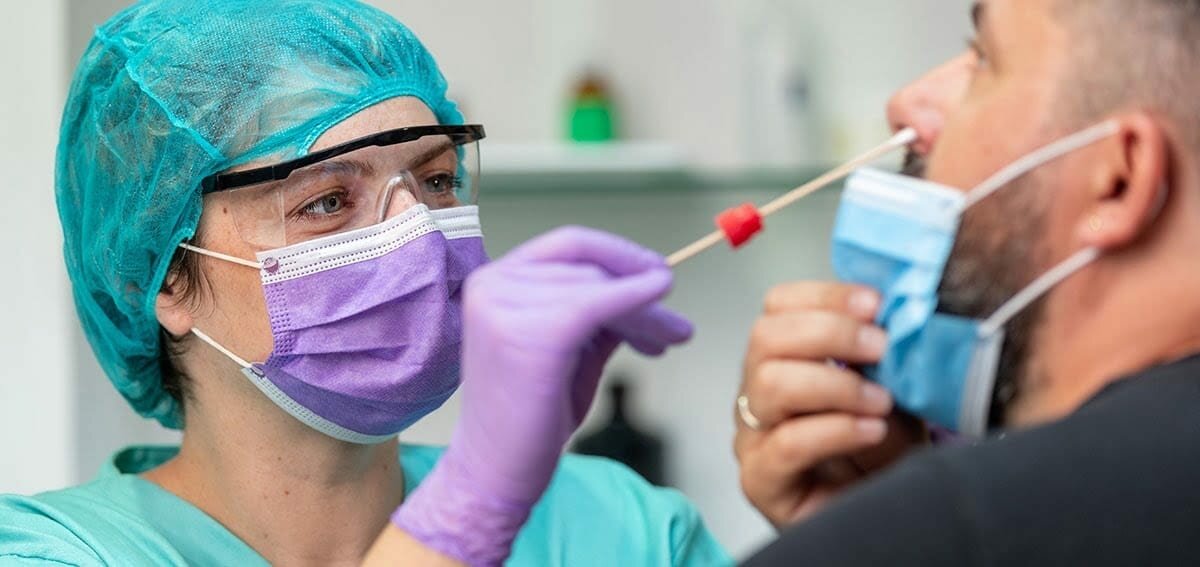
I'm in the middle of an intensive conversation around mrna with a friend, who wants to see the previous studies around animals particularly ferrets and mrna tests, when reintroduced to the virus.
But, typically, can I find the paper.?? Nope!
Typical when I save so much, I can't find this one..
Can I call for aid!
Thanks all
I think this is what you are looking for.
Background Severe acute respiratory syndrome (SARS) emerged in China in 2002 and spread to other countries before brought under control. Because of a concern for reemergence or a deliberate release of the SARS coronavirus, vaccine development was initiated. Evaluations of an inactivated whole...

journals.plos.org
This is found near the end of the study.
This combined experience provides concern for trials with SARS-CoV vaccines in humans. Clinical trials with SARS coronavirus vaccines have been conducted and reported to induce antibody responses and to be “safe”
[29],
[30]. However, the evidence for safety is for a short period of observation. The concern arising from the present report is for an immunopathologic reaction occurring among vaccinated individuals on exposure to infectious SARS-CoV, the basis for developing a vaccine for SARS. Additional safety concerns relate to effectiveness and safety against antigenic variants of SARS-CoV and for safety of vaccinated persons exposed to other coronaviruses, particularly those of the type 2 group. Our study with a VLP SARS vaccine contained the N protein of mouse hepatitis virus and Bolles, et al., reported the immunopathology in mice occurs for heterologous Gp2b CoV vaccines after challenge
[25]. This concern emanates from the proposal that the N protein may be the dominant antigen provoking the immunopathologic reaction.
Because of well documented severity of the respiratory disease among infants given an inactivated RSV vaccine and subsequently infected with RSV that is considered to be attributable to a Th2-type immunopathologic reaction and a large number of studies in the Balb/c mouse model that have described and elucidated many components of the immunopathologic reaction to RSV vaccines, the similarity to the SARS-CoV vaccine evaluations in Balb/c mice supports caution for clinical vaccine trials with SARS-CoV vaccines in humans. Of interest are the similar occurrences in C57BL/6 mice and in ferrets and nonhuman primates that provide alternative models for elucidating vaccine-induced mechanisms for occurrences of Th2 immunopathologic reactions after infection. As indicated, strong animal model evidence indicates expression of the N protein by SARS-CoV vector vaccines can induce sensitization leading to a Th2–type immunopathology with infection. In contrast to our results, those studies did not find clear evidence of the Th2 type immunopathology on challenge of mice given a vector vaccine for the S protein. The finding of a Th-2-type pathology in our studies in animals immunized with an rDNA-produced S protein is unequivocal. In this regard, animal model studies with FIPV in cats and RSV in mice have indicated that viral surface proteins may be the sensitizing protein of inactivated vaccines for immunopathology with infection
[32],
[45]. This suggests that presentation of the S protein in a vector format may direct immune responses in a different way so that sensitization does not occur.
Limitations of the present studies include their performance in mice only and uncertainty of the relevance of rodent models to SARS-CoV vaccines in humans. Additionally, a more intense study for virus replication including quantitative RT-PCR assays might have confirmed the probability that virus replication is required for induction of the immunopathology after vaccination. Evaluations of mechanisms for the immunopathology, including immunoglobulin and cytokine responses to vaccines and tests for antigen-antibody complexes in tissues exhibiting the reaction, could have strengthened the Th2-type immunopathology finding. Finally, a successful study with a Th1-type adjuvant that did not exhibit the Th2 pathology after challenge would have confirmed a Th2 bias to immune responses as well as provide a potential safe vaccination approach for SARS.

www.dailymail.co.uk





